1.
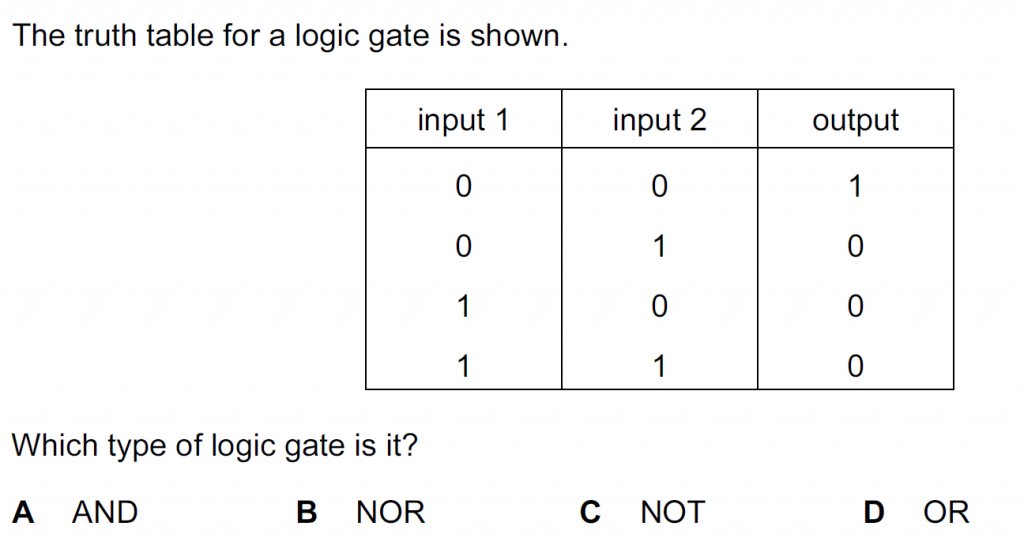
Answer : A
Solution
The AND gate will be ON or give a bit 1 as an output if both the inputs are ON or 1. So,
2.
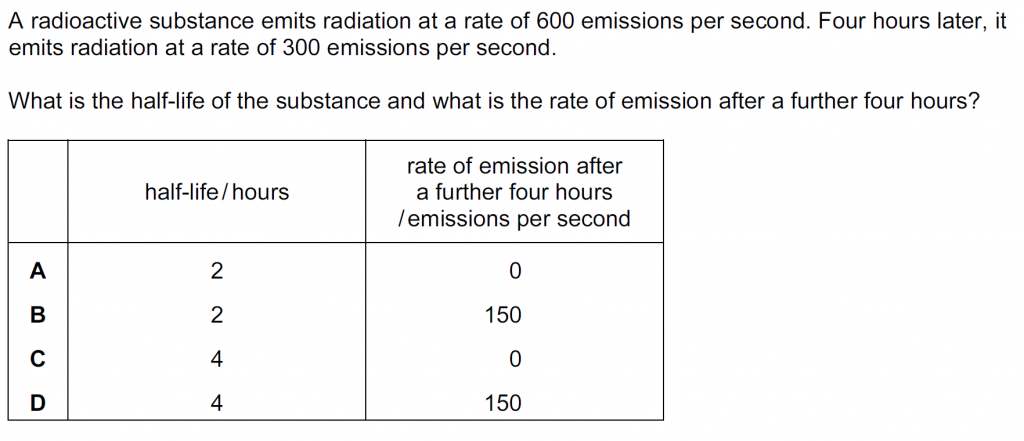
Answer : D
Solution
The time in which the activity halves is the half-life, which is 4 hors here as the activity halves from 600 emissions per second to 300 emissions per second in 4 hours. In another four hours it will half again to 150 emissions per second.
3.
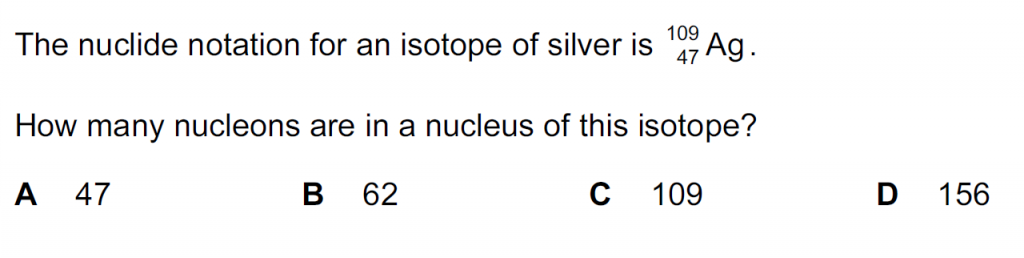
Answer : C
Solution
Nucleon number is indicated by the superscript which here is 109.
4.
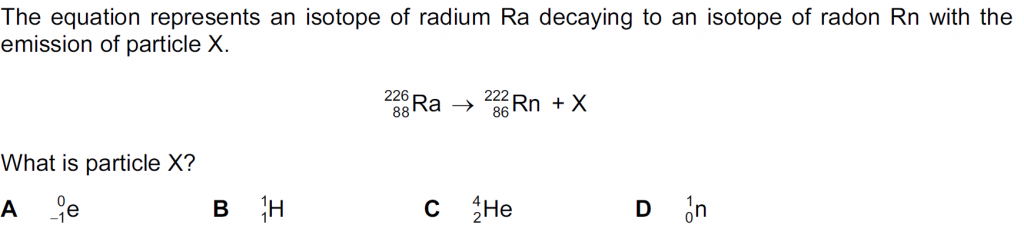
Answer : C
Solution
Particle X has a nucleon number of 4 and a proton number of 2, as balancing the superscripts and the subscripts shows, so it is an alpha particle, or a Helium nucleus. So,
5.
Answer : A
Solution
An alpha decay will lead to proton number decreasing by 2, and then the beta decay will lead to the proton number increase by 1, so the net result is a decrease in proton number by 1.
6.

Answer : A
Solution
The particle has a nucleon number of 0, and a charge of -1, so it is a beta particle.
7.
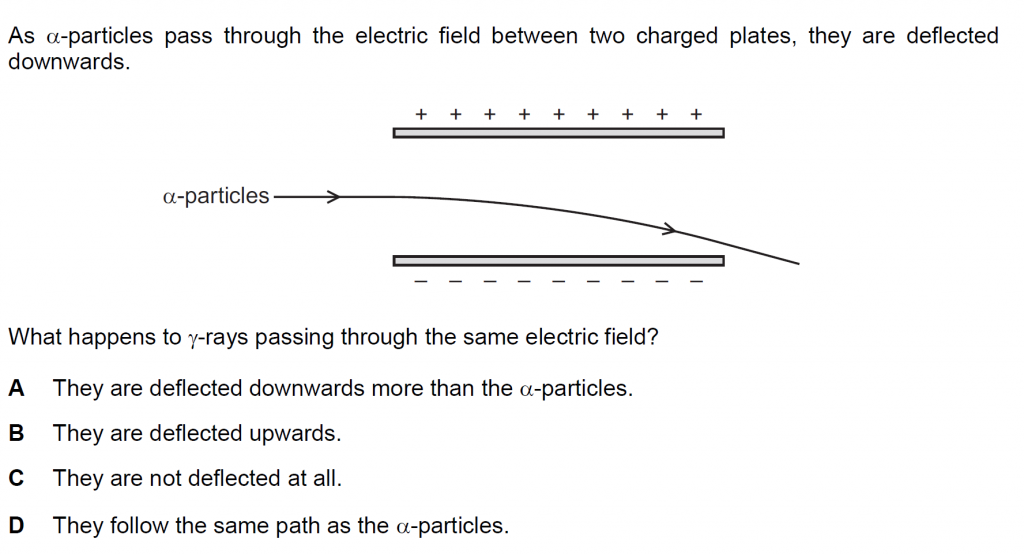
Answer : C
Solution
The gamma rays are high frequency uncharged electromagnetic radiation and are not deflected at all by the electric field between the two charged plates.
8.

Answer : D
Solution
In a half-life of 8 days, the number of iodine- 131 will reduce to half. In another 8 days, it will further reduce by half, thus it will reduce to one quarter in 16 days.
9.
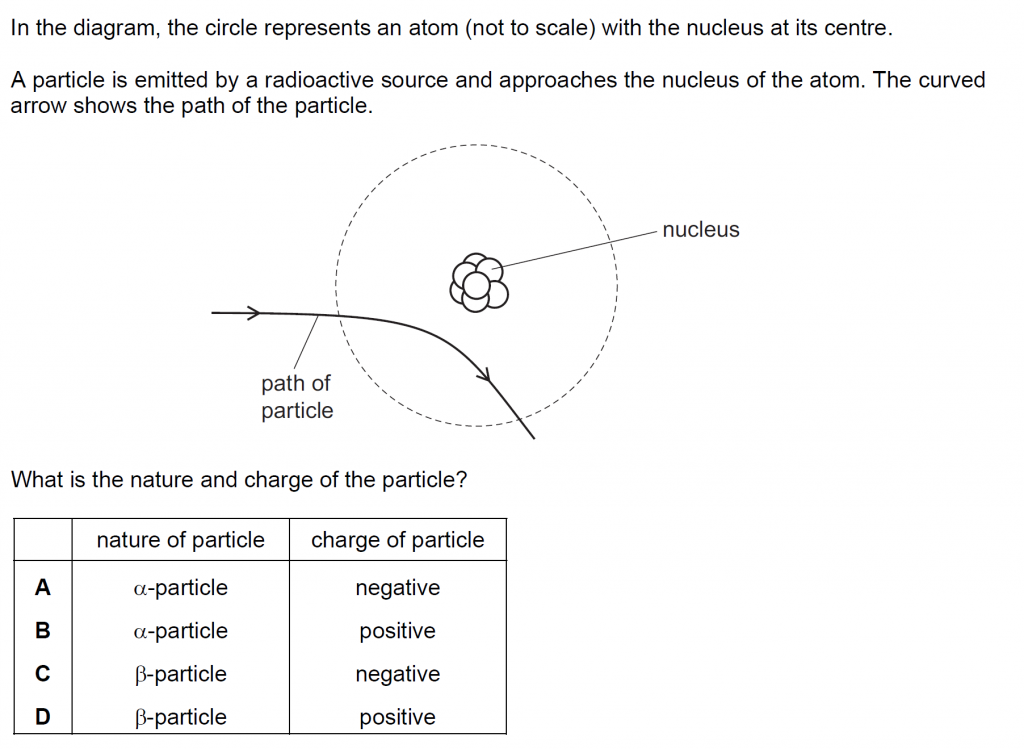
Answer : B
Solution
As it is repelled by the positive nucleus it must be positive itself. The nature is therefore alpha, and charge, positive. Beta is negative, so it can’t be as it will then be attracted.
10.
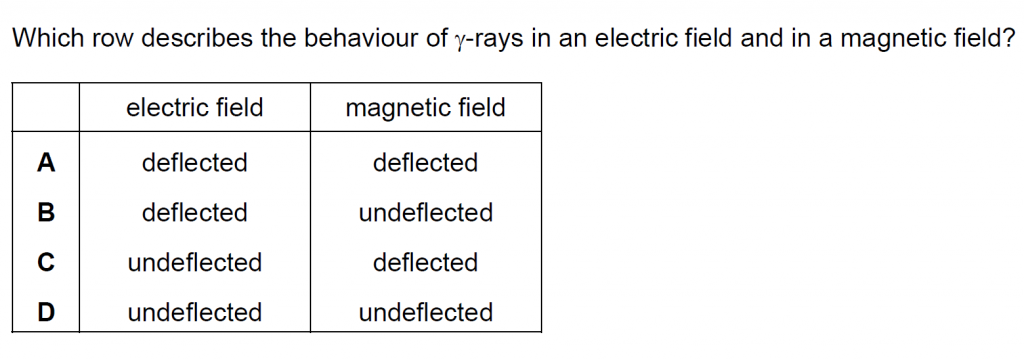
Answer : D
Solution
Gamma rays being uncharged are neither deflected by electric field nor by magnetic field.
11.

Answer : A
Solution
1.5 hours is three times the half-life of 0.5 hours, thus its activity will decrease by a factor of 8, and become, 6000/8 = 750 counts per second.
12.

Answer : A
Solution
The fact that only a few alpha particles are deflected by large angles and come straight back from the foil towards the source provides the evidence of a small charged nucleus.
13.

Answer : A
Solution
A neutral atom of copper has the structure of a nucleus surrounded by electrons.
14.

Answer : A
Solution
Radioactive decay is a random, spontaneous process, and therefore it is impossible to tell which nuclei will decay first.
15.
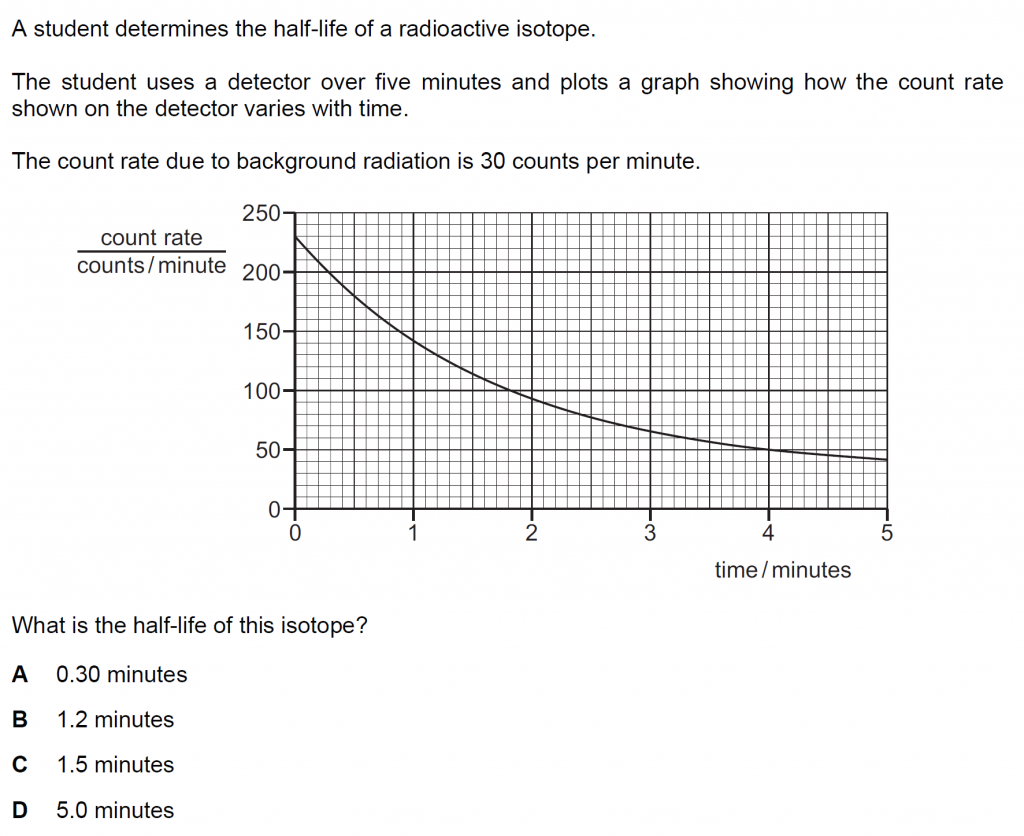
Answer : B
Solution
Because of a background radiation of 30 counts per minute, we can reduce the count rate by 30. Thus, at t = 0 minutes, the count rate is 230 – 30 = 200 counts/ minute. In one half-life, this will reduce to 100 counts/ minute, and the detector will show, 100 + 30 = 130 counts/ minute.
This happens at, 1.2 minutes.