Radioactivity
Background Radiation
- Traces of radiation are present all around us.
- It is not harmful, and it cannot pass through our skin.
- Its activity varies from 20 to 40 counts/second.
- Sources of this radiation are outer space (cosmic radiation), nuclear tests and reactors, radioactive rocks, some medicines, and some food.
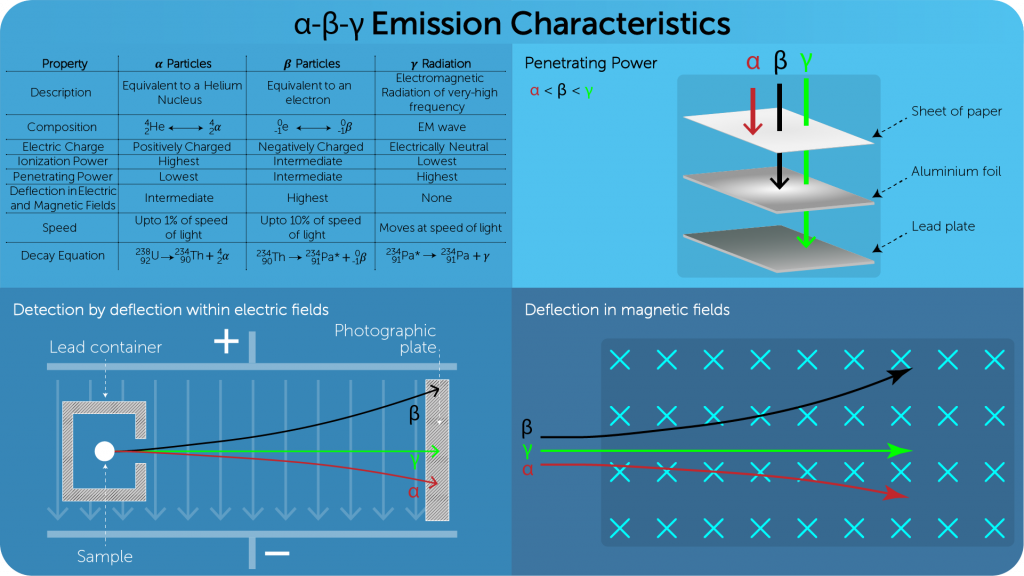
Types of Radiation
- Alpha (
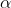 ): Helium Nucleus, positively charged:
): Helium Nucleus, positively charged: 
- Beta (
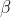 ): Electrons, negatively charged:
): Electrons, negatively charged: 
- Gamma (
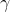 ): High frequency electromagnetic radiation
): High frequency electromagnetic radiation
- The particles are emitted in nuclear reactions (fission, fusion, and artificial transmutation) and in radioactive decay.
- The spontaneous emission of alpha (
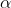 ), beta (
), beta (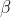 ), and gamma (
), and gamma (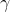 ) particles, independent of temperature and pressure, by radioactive nuclei is called radioactive decay.
) particles, independent of temperature and pressure, by radioactive nuclei is called radioactive decay.
- This emission leads to increasing the stability of radioactive nuclei.
- Emissions are random, so the order of emissions of these particles can only be predicted with probabilities.
5.2.2 Radioactive Emissions
 emission Uses
emission Uses
 Particles:
Particles:
- Smoke detection
- Radioactive materials release -particles which ionizes the air inside the detector.
- Smoke from a fire absorbs -radiation altering the Ionization and triggering the alarm.
 Particles:
Particles:
- Doctors use radioactive chemicals tracers for medical imaging.
- An isotope that emits particles can be injected in the bloodstream and its motion can be monitored by using a detector outside the body.
- Accumulation of the particles at any point in the blood stream will indicate a blockage.
- particles are used in detectors that monitor and control the thickness of materials such as paper, plastic, and aluminum.
- The thicker the material, the more radiation is absorbed, and less radiation reaches the detector.
- It then sends signals to the equipment that adjust the thickness of the material.
 Radiation:
Radiation:
- Treatment of cancer
- Testing Equipment
- Sterilizing medical equipment
- Crack detection in metallic pipes
5.2.3 Radioactive Decay and Half Life
- Heavy radioactive isotopes decay into lighter isotopes of higher stability until they decay into lead, which is the most stable element.
- The time it takes for half of a sample of radioactive isotopes of an element to decay is known as the Half Life ( ).
- This yields an exponential graph of radioactive decay.
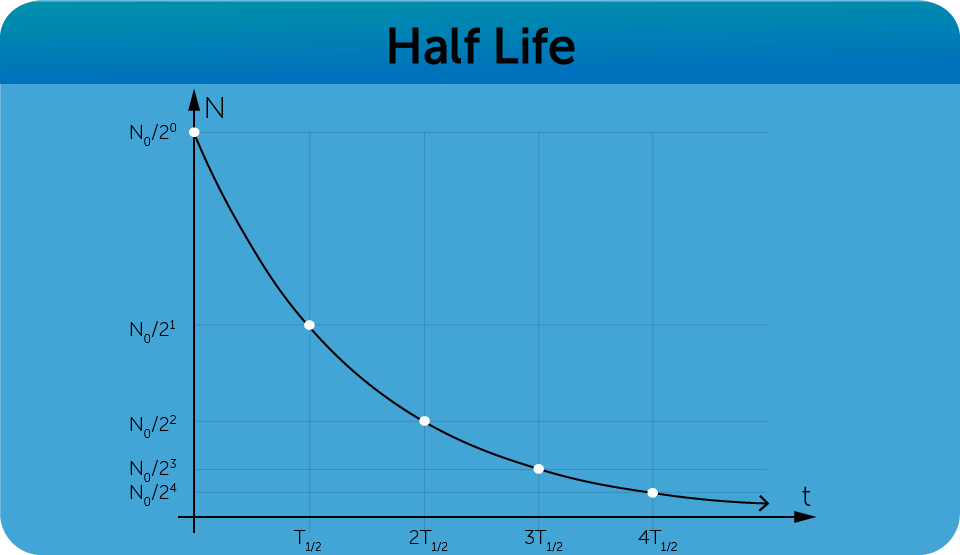
 = original number of nuclei or activity at time
= original number of nuclei or activity at time 
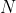 = number of nuclei at time
= number of nuclei at time 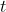
 = number of nuclei at time
= number of nuclei at time 
- Number of nuclei after n half-lives

5.2.5 Safety Precautions
- Wearing protective clothing.
- Keep as far away as practical, e.g. use tongs.
- Keeping your exposure time as short as possible.
- Keeping radioactive materials in lead-lined containers, labelled with the appropriate hazard symbols.
Waste
- Low-Level radioactive waste, such as contaminated gloves, can be disposed of in landfill sites.
- But higher-level waste, which may be dangerously radioactive, is difficult to dispose of.
- It can be reprocessed to extract nuclear fuel or encased in lead and left deep underground.
Precautions with usage of Radioactive Isotopes
- Distance – tongs
- Absorption – lead gloves, protective suit, goggles
- Time – limit exposure time

emission Uses
Particles:
Particles:
Radiation:
