1.
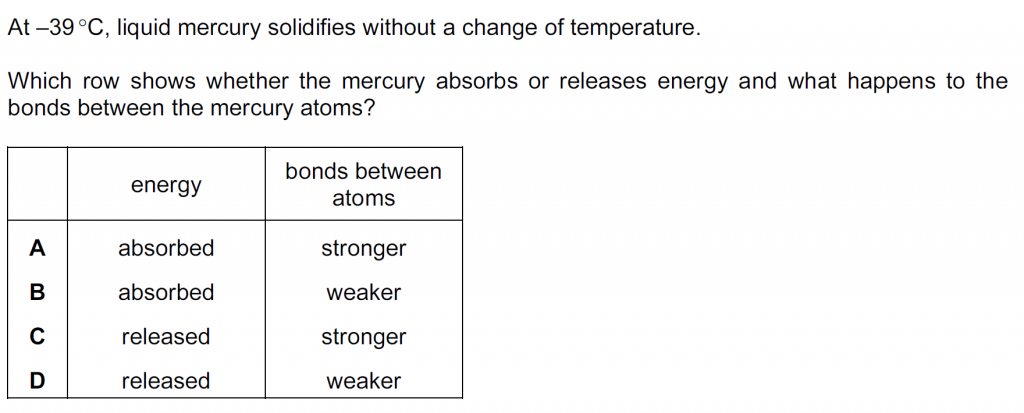
Answer : C
Solution
As liquid mercury solidifies it releases energy, as energy is required to be given to a solid to melt it. Also, the intermolecular separation decreases when a liquid becomes a solid, because of which the bonds between atoms becomes stronger.
2.
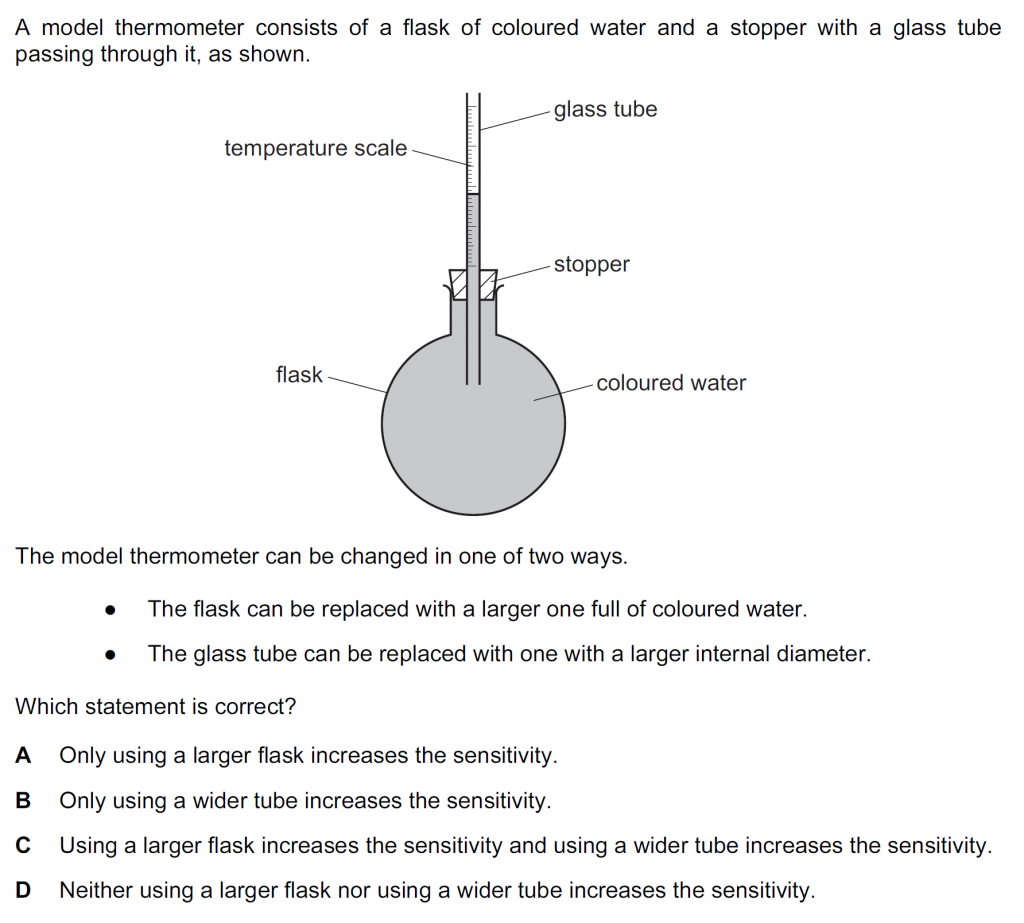
Answer : A
Solution
When we use a larger flask the volume of the coloured water increases, so the expansion or increase in volume increases for the same temperature change, so the rise in water level in the glass tube increases making it more sensitivity. The increase in the internal diameter of the glass tube will decreases the increase in the level of water in the tube for a temperature change. Thus, will not result in an increase in sensitivity. So,
3.

Answer : C
Solution
The thermal capacity of an object = mass x specific heat capacity = 2.0 x 360 = 720 J/ oC.
4.

Answer : C
Solution
Free electrons are present in large numbers in metals only where they are involved in thermal energy transfer. All solids don’t have a plethora of free electrons.
5.
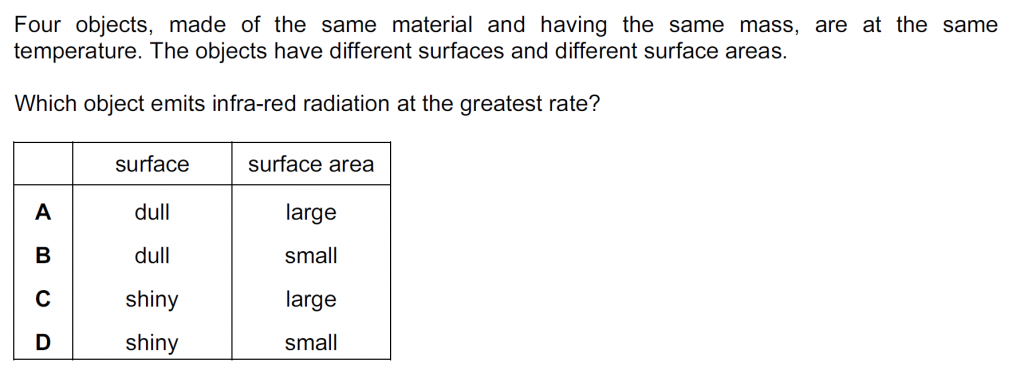
Answer : A
Solution
Dull surfaces absorb and emit infra-red radiation at the greatest rate, and larger surface areas emit at the greatest rate. Also, shiny surfaces reflect the most and absorb and emit at the slowest rate.
6.

Answer : B
Solution
Pressure is exerted due to the collisions of molecules with the container walls. This is because they undergo a change in momentum on collisions due to impulse exerted by the wall on the molecules and an equal and opposite impulse exerted by the molecules on the walls. This leads to a force and thus a pressure on the wall.
7.
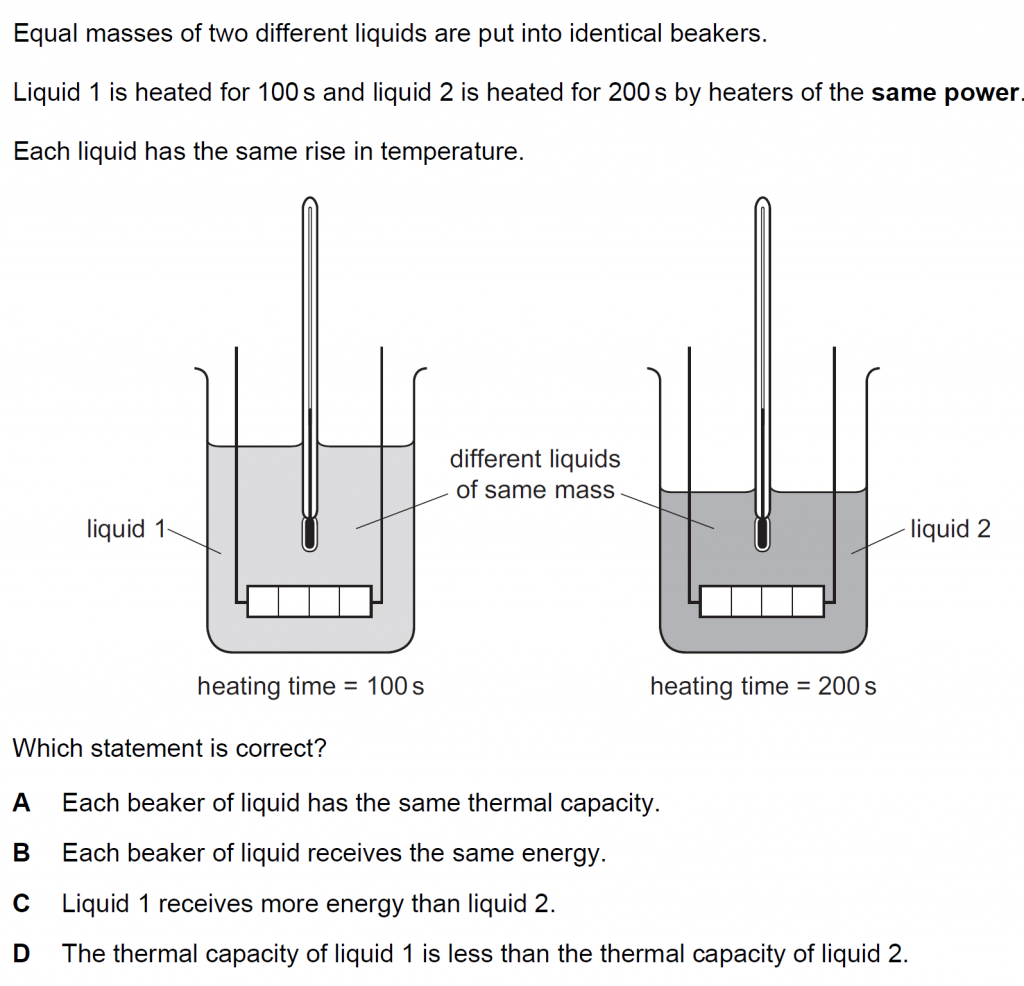
Answer : D
Solution
Heat input to liquid 2 is twice that of the heat input to liquid 1. The masses are the same, and the rise in temperature is the same. This is because the specific heat capacity of liquid 1 is less than that of liquid 2, so the thermal capacity of liquid 1 is less than the thermal capacity of liquid 2.
8.

Answer : C
Solution
Q = m L = 100 g x 2300 J/g = 230000 J = 230 000 J
9.
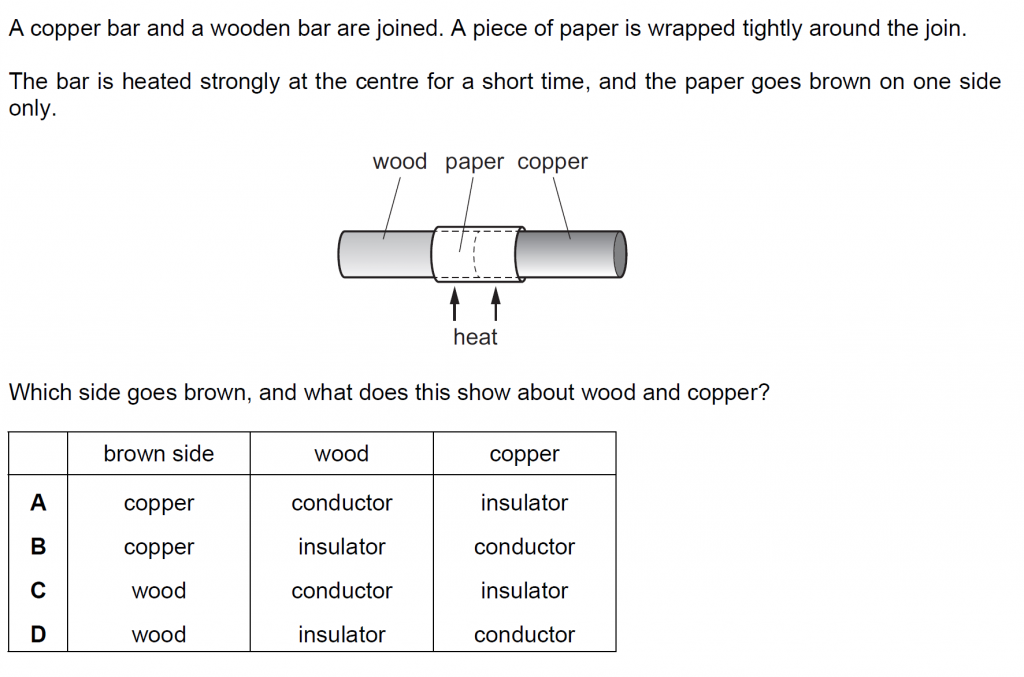
Answer : D
Solution
The side, which is unable to transfer heat goes brown, which of course is wood. Copper on the other hand is a conductor and conducts heat away.
10.

Answer : D
Solution
The molecules of the liquid evaporate quickly due to air being blown and this results in cooling as the most energetic molecules leave the liquid. Temperature being a measure of average kinetic energy, reduces as the most energetic molecules leave the liquid body.
11.

Answer : A
Solution
Energy needed to melt a unit mass of ice into water at its constant melting temperature.
12.
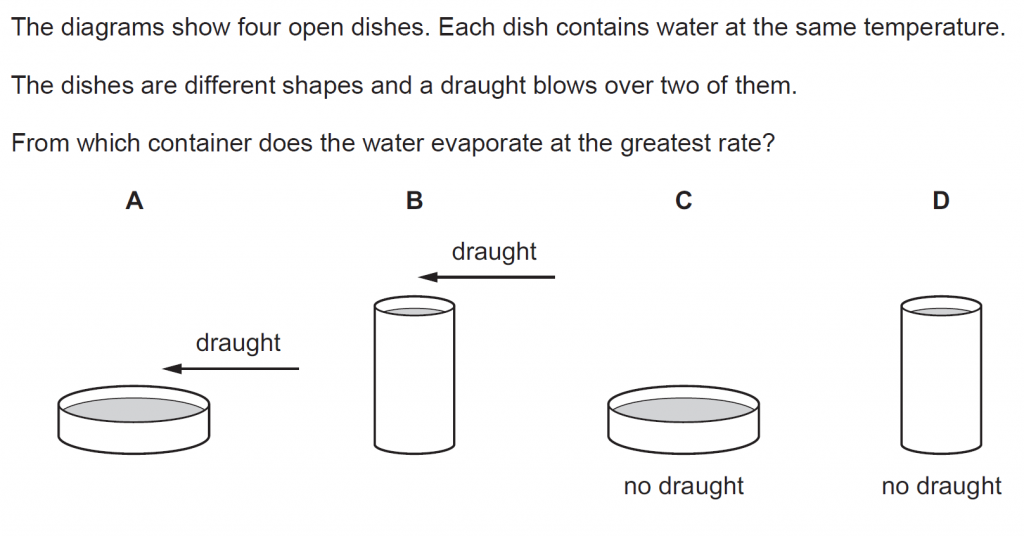
Answer : A
Solution
Rate of evaporation increases with an increase in surface area and if there is a draught blowing over it.
13.
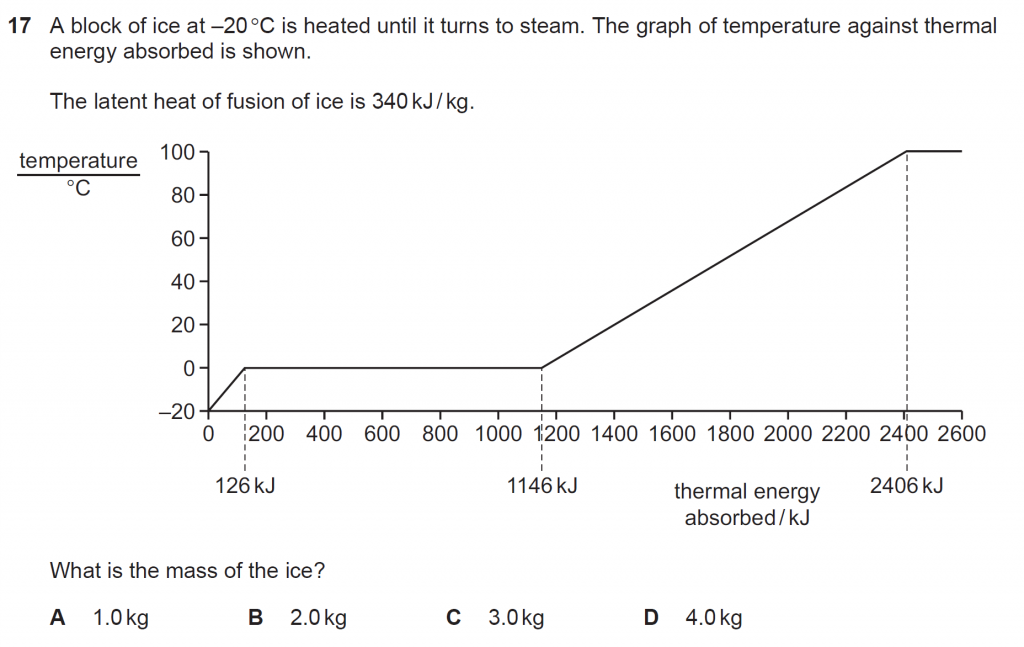
Answer : C
Solution
Heat required to melt a mass m of ice into water of mass m at its melting point of 0o C is Q = m L.
Q = 1146 kJ – 126 kJ = m 340 kJ/kg
m = 1020/340 = 3.0 kg
14.
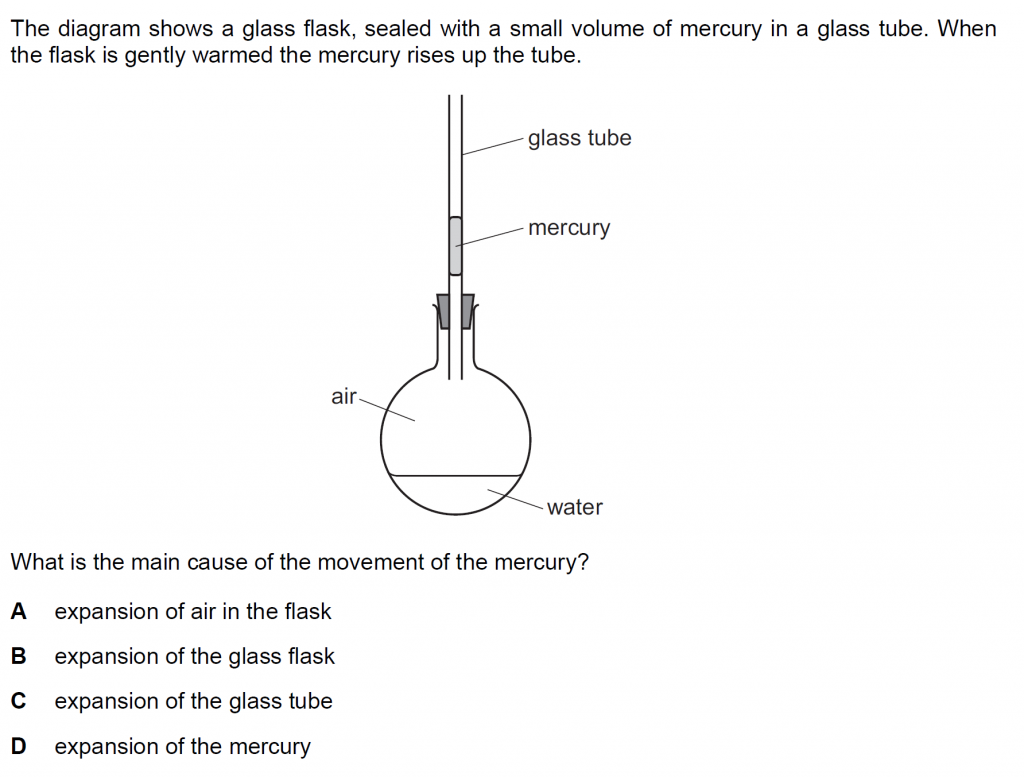
Answer : A
Solution
The mercury rises because the air in the flask expands and pushes the mercury upward.
15.
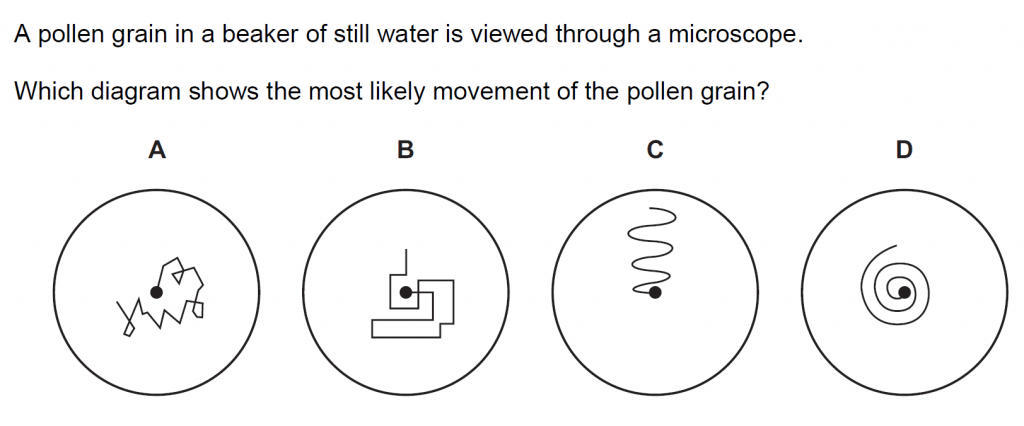
Answer : A
Solution
The pollen grains are bombarded randomly from all sides by the water molecules making them bounce around randomly.
16.
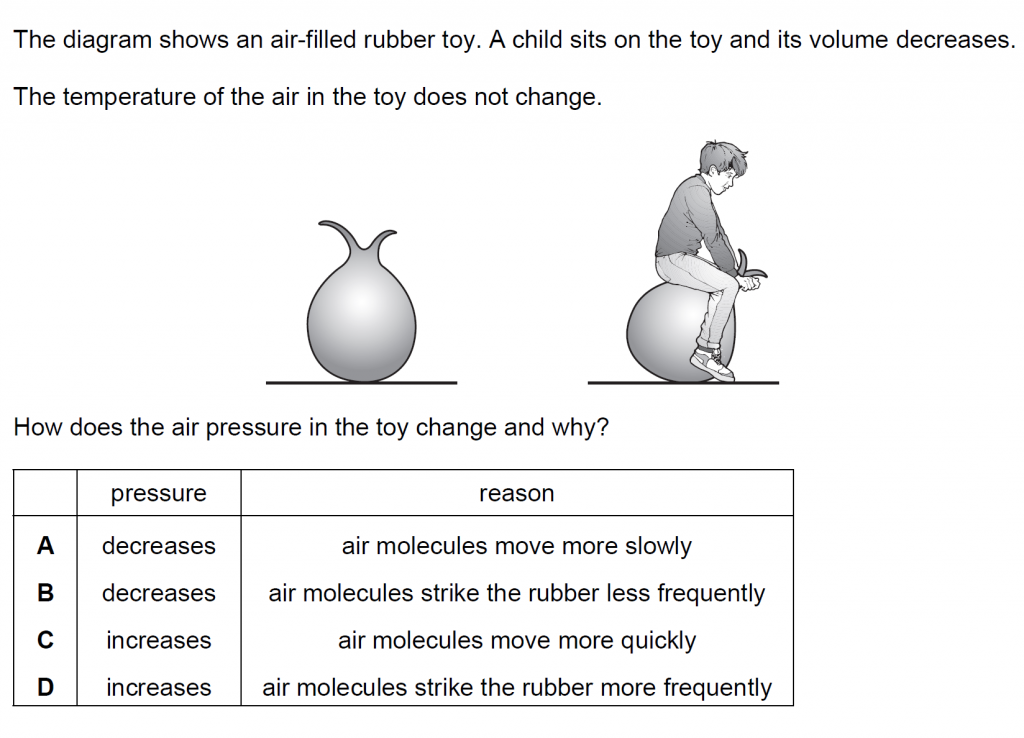
Answer : D
Solution
Because of the decrease in volume, the air pressure increases because of more frequent collisions with the rubber.
17.
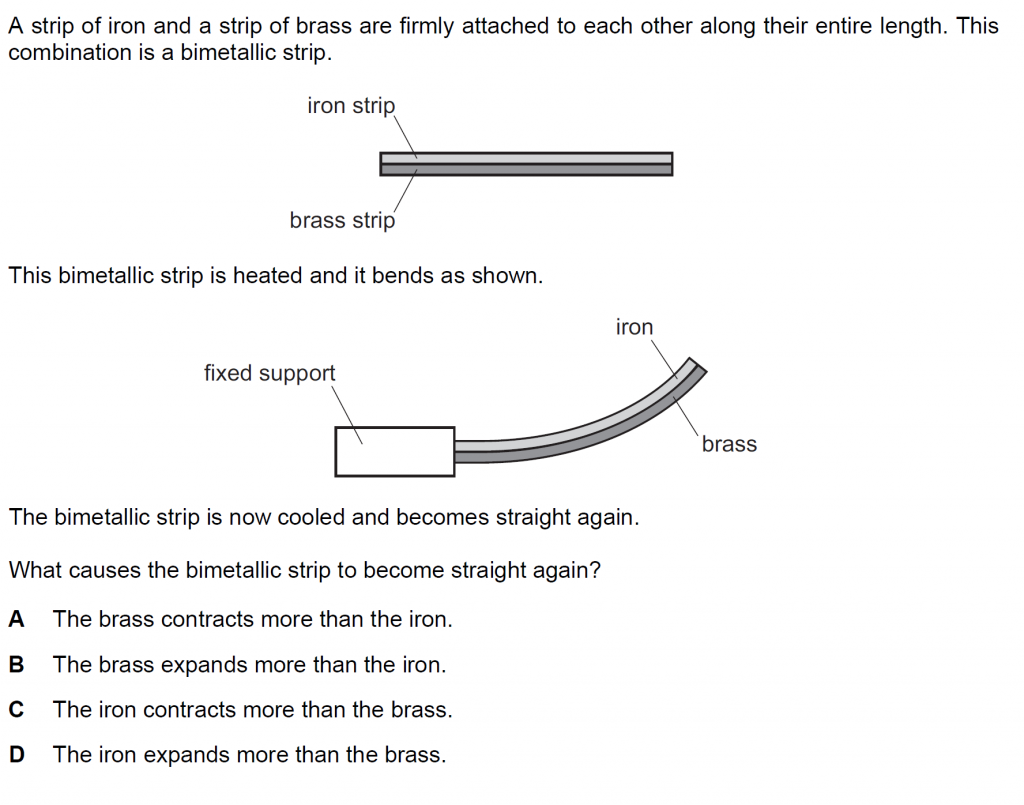
Answer : A
Solution
The brass contracts more than iron, thus straightening out the bimetallic strip.
18.

Answer : C
Solution
Q = m c ΔT
Q = 0.200 x 900 x (110 – 20)
Q = 16200 J
19.

Answer : A
Solution
When cooled, volume of liquids decreases and they become denser and move downward, heating up the entire liquid body. Convection currents occur in liquids and gases.
20.
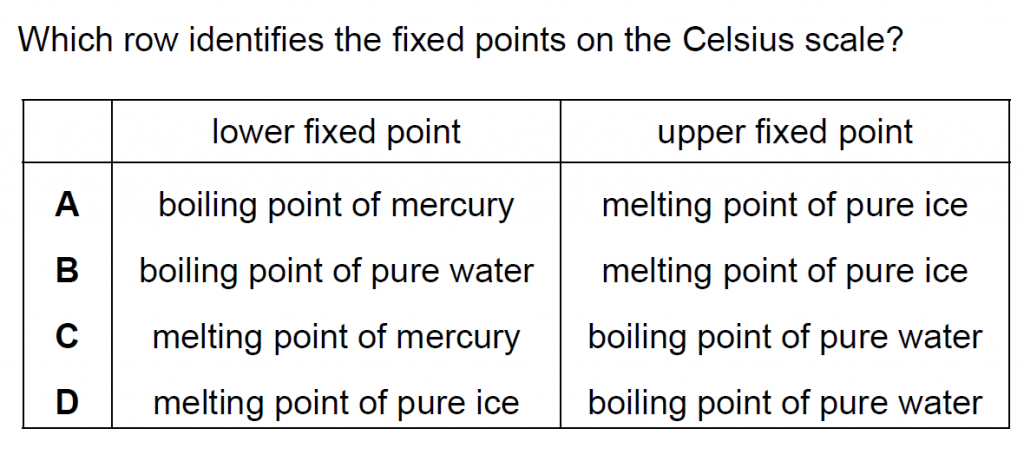
Answer : D
Solution
Melting point of pure ice is the lower fixed point, fixed at 0oC and boiling point of pure water is the upper fixed point, fixed at 100oC.
21.
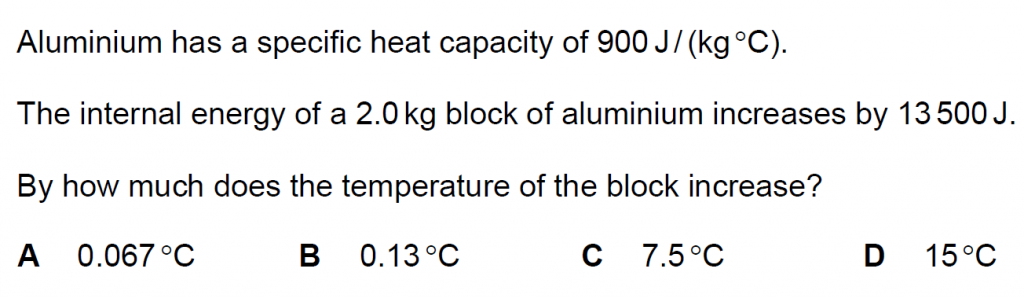
Answer : C
Solution
Q = m c ΔT
13500 = 2.0 x 900 x ΔT
ΔT = 13500/ 1800 = 7.5 oC.
22.

Answer : B
Solution
Hot air is less dense than cold air, so the hot air balloon rises.
23.

Answer : A
Solution
Half-life of a radioactive isotope does not depend on the temperature so cannot be used for the measurement of temperature.
24.
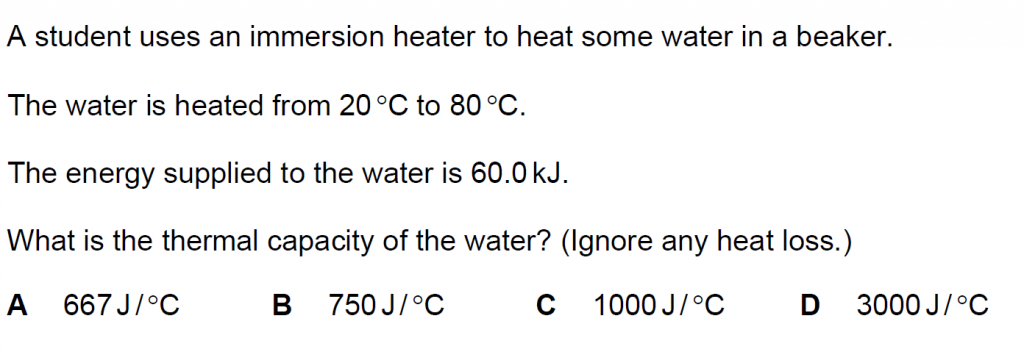
Answer : C
Solution
Q = m c ΔT
Thermal capacity is mass x specific heat capacity
C = m c
Q = C ΔT
C = 60.0 kJ/ (80 – 20) oC = 60.0 kJ/ 60oC = 1000 J/ oC
25.

Answer : C
Solution
The water at the bottom of the tank heats up and rises as it becomes less dense than cold water, thus heating up the entire liquid body by setting convection currents.