States of Matter:
- All matter is made up of atoms/ molecules which are in constant random motion.
- The random kinetic energies of molecules increase with an input of thermal energy, due to a temperature difference or due to mechanical work being done on it.
- Intermolecular force of attraction between molecules in Solids is >liquids > gases.
- Intermolecular space in gas > liquid > solid.
- Kinetic energy per molecule increases with increase in temperature in all solids, liquids & gases.
- With change in temperature, change in volume in gas is > liquid > solid.
- In solids, molecules are in lattice form; they can vibrate and rotate about their mean position.
- In liquids, molecules co-exist in groups. They can slide over each other and they can move from one point to another in groups.
- In gases, molecules can move independently, with negligible intermolecular forces.
- Internal energy of an object is the sum of kinetic and potential energies of all the molecules making up the body.
- On the input of energy the kinetic and potential energies increase, except when there is a phase change, when the energy input goes into increasing the potential energies of the molecules, rather than increasing the kinetic energies, which is why the temperature does not show a change when there is a phase change going on.
Temperature:
- Average kinetic energy per molecule of a substance is the measure of its temperature in kelvin.
Absolute zero:
It is the temperature at which average kinetic energy per molecule of a substance is zero. As the object is heated the kinetic energies increase, with an input of thermal energy, with a removal of thermal energy, there is a decrease in kinetic energy and therefore temperature. This shows that with a decrease in temperature there is a decrease in kinetic energy and there will be a temperature at which the kinetic energies will drop to 0 Joules, and that will be the lowest temperature possible, which we call as . There cannot be a lower temperature as the kinetic energies cannot become negative.

Brownian Motion:
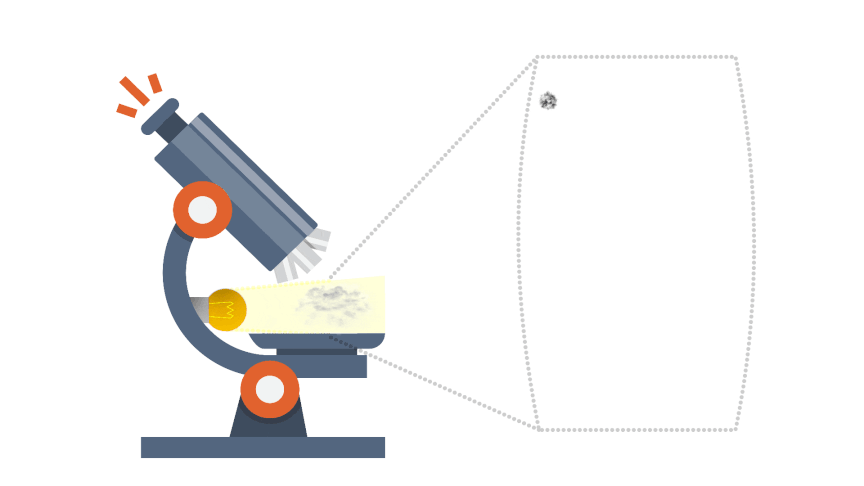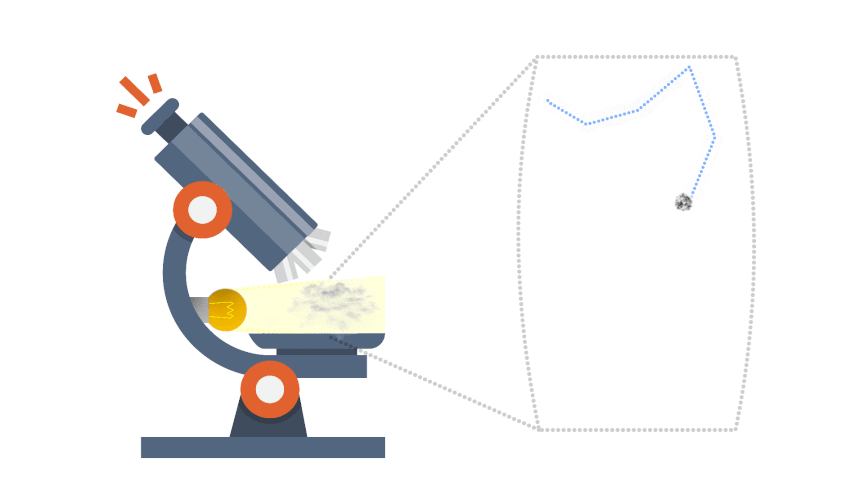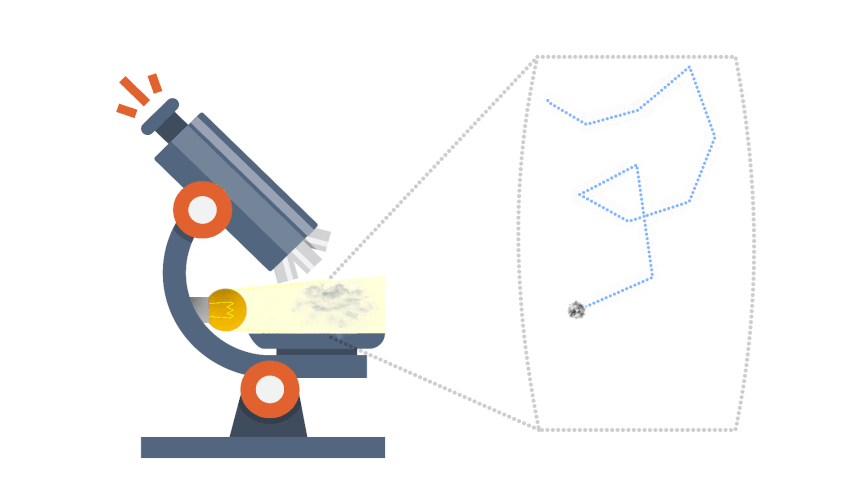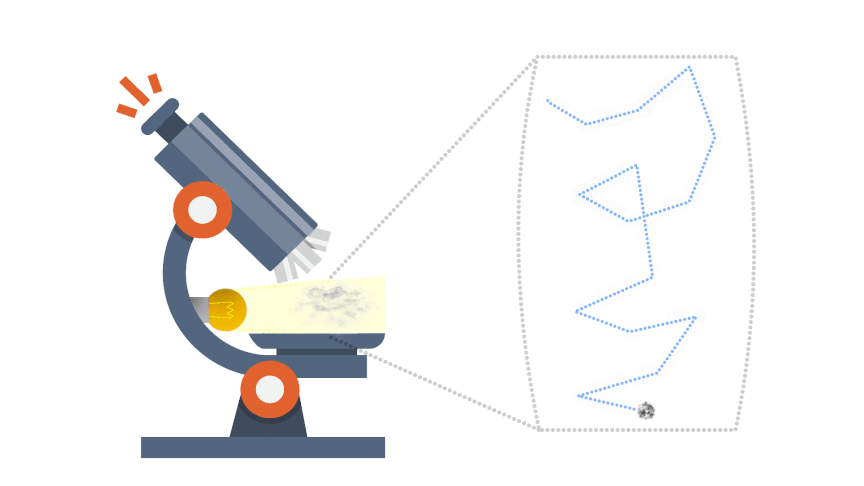
Brownian motion gives indirect evidence for the existence of atoms and molecules. Some smoke is introduced into a transparent container containing air. The smoke particulars appear as spots of light when illuminated and viewed through a microscope.
The smoke particles are seen to move in very erratic, irregular jiggly manner; there motion is due to being repeatedly hit by invisible molecules of the air, from all directions and producing a net unbalanced force, which would be both random in direction and magnitude.
Evaporation:
- Evaporation occurs when molecules escapes from a liquid without the liquid reaching its boiling point.
- In terms of kinetic theory, a pool of water contains many molecules of liquid water in constant motion.
- The molecules near the surface can actually jump out of the liquid on colliding with molecules from below and get knocked out into a vapor form, as they escape to become vapour molecules by overcoming intermolecular force of attraction.
- This is called evaporation.
- It is a surface phenomenon.
- Characteristics of Evaporation
- It occurs at any temperature.
- Rate of evaporation increases with increase in temperature.
- Rate of evaporation increases with increase in surface area.
- Rate of evaporation is directly proportional to the speed of the wind blowing over it.
- Rate of evaporation would decrease if the humidity of the atmosphere is more.
- Evaporation accompanies the cooling effect of the liquid, as the most energetic molecules leave the body of the liquid, the rest of them have a lower average kinetic energy, and therefore a lower temperature, that is it cools down.
Pressure of a gas:
- It is the force acting per unit area by gas molecules on the wall of a container.
- It is also defined as the total rate of change of momentum of all the molecules of a gas to the total surface area of the wall.
- As the change in momentum on a collision is 2mv, it will be larger if the impact speeds v are higher, and also the rate of change of momentum will increase if the number of collisions per unit time, that is frequencies of collisions increase.
- This will increase the force exerted, from Newton’s second law, and therefore the pressure.
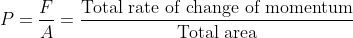
Equation of state: