Energy: Potential and Kinetic
- Energy is defined as the capacity of a body to do work.
- It is a scalar quantity.
- SI unit is Joules, J.
- A body can possess energy in various forms including, but not limited to:
- Gravitational Potential Energy
- Kinetic Energy
- Chemical Energy
- Elastic (Strain) Energy
- Nuclear Energy
- Internal Energy
Gravitational Potential Energy
- It is the energy possessed by an object due to its position and configuration

-
- where PE is the Potential Energy in J
- m is the mass of the object in kg
- g is the acceleration due to gravity,
 on the surface of the Earth
on the surface of the Earth
- h is the height above the ground
- When an object is raised or lowered, it gains or loses gravitational potential energy.
- This change is given by the following equation:

- where
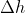 is the change in height of the object
is the change in height of the object
- Note how it’s only the change in height that affects the gravitational potential energy, not horizontal movement
Kinetic Energy
- An object in motion possesses Kinetic energy that depends on its mass and speed.

- where KE is the Kinetic Energy in J
- m is the mass of the object in kg
- v is the speed of the object in m/s
- Note that the Kinetic energy depends a lot more on the speed of the object rather than the mass of the object.
Energy: Other forms
Chemical Energy
- Any energy that is contained and can be released by chemical reactions is known as Chemical energy.
- Examples –
- Batteries
- Fossil Fuels
- Muscles in our bodies
Elastic (Strain) Energy
- Whenever a body is compressed or extended, it is said to contain elastic energy.
Example: extension of a spring
- In the subunit Forces topic Hooke’s Law, we learned about the Force versus Extension graph for a spring.
- The area under the force-extension graph is the elastic energy contained by the spring.

- where EE is the elastic energy in J
- F is the force exerted to extend the spring in N
- x is the extension in m
Nuclear energy
- When a highly energetic neutron collides with a Uranium nucleus, it splits into daughter nuclei.
- Energy is released as part of this process called the Nuclear Fission Reaction.
- The energy released is called Nuclear Energy.

- This reaction takes place in a nuclear reactor.
- An explosion of an atomic bomb also releases nuclear energy.
- the reaction is controlled in Nuclear Reactors, while in atomic bombs the reaction is uncontrolled.
- Contrastingly, Nuclear Energy is also released by the process of a Nuclear Fusion Reaction.
- The most well-known Fusion reaction takes place at the core of the sun.
- In this reaction, Hydrogen atoms combine to form a helium nucleus release a tremendous amount of energy.
- Unfortunately due to the strong forces of repulsion between the protons of the nucleus, a tremendous amount of energy is required to get the reaction started.
- Thus, replicating the Nuclear Fusion reaction is extremely difficult to perform artificially.
Internal Energy (Heat)
- In a substance, atoms rotate and vibrate with some energy called internal kinetic energy.
- Internal potential energy depends on the separation between atoms in a substance.
- Internal energy is the sum of these Internal kinetic and Internal potential energies.
- With increase in temperature both internal kinetic energy and potential energy increase.
- Therefore, internal energy increases with an increase in temperature.
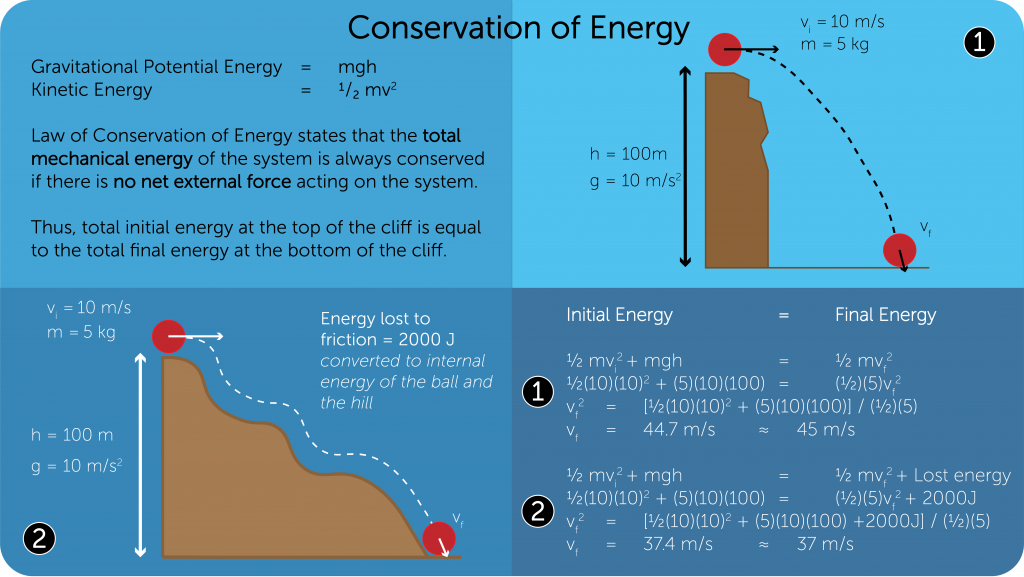
Law of Conservation of Energy
- Energy can neither be created nor be destroyed, but it can be converted from one form to another.
- The following are a number of examples that showcase the transfer of energy as a ball falls off a hill.
Energy Resources and Efficiency
- In order to obtain useful energy such as Electrical Energy, it must be converted from some form it already exists in into Electrical energy.
- Example –
- Chemical Energy (Fossil Fuels)
- Potential and Kinetic Energy in Water (Tides, Hydroelectric Dams)
- Heat Energy in Natural Gasses (Geothermal)
- Nuclear Energy (Fission)
- Heat and Light Energy (Sun)
- Kinetic Energy in the Wind
- Note how the Sun is the original source of nearly all these forms of Energies except for Geothermal, Nuclear, and Tidal.
Chemical energy
- Chemical energy is stored in fuels such as Diesel, Petrol, and Coal
- These fossil fuels are burned in order to heat water.
- The steam is pushed through a turbine that rotates to produce electricity.
Potential and Kinetic Energy in Water (Waves, Tides, Hydroelectric Dams)
- The Kinetic energy from water waves can be used to push turbines in order to generate electricity.
- In case of Tidal and Hydroelectric Dams, the water is collected behind a barrier that allows it to gain Gravitational Potential energy.
- It is then slowly released and the Potential energy transforms into Kinetic energy as the water is then rushed through a turbine to generate electricity.
Heat Energy in Natural Gasses (Geothermal)
- Natural gasses are trapped in high pressure areas at high temperatures under the surface of the Earth.
- The heat from these gasses can be used to heat water, and similar to fossil fuels, the steam runs through a turbine to spin it and generate electricity.
Nuclear Energy in Radioactive Materials (Fission)
- The energy released by a Nuclear Fission Reaction can be used to boil water.
- The steam is then used to rotate a turbine to generate electricity.
Heat and Light Energy (Sun)
- Heat from the Sun can be converted to electricity by Solar Thermal Panels.
- Light from the Sun can be converted to electricity using Photovoltaic Solar Cells.
Kinetic Energy in the Wind
- In Windmills, the kinetic energy of wind gets converted to mechanical energy
- This mechanical energy is used to rotate a turbine to generate electricity.
Energy and Power Efficiency
- Energy and Power efficiency is defined as the percentage of useful energy or power output from a certain amount of energy or power input.


Renewable and Non-Renewable Energy
| Renewable Energy |
Non-Renewable Energy |
| Examples:
· Wind
· Solar
· Tidal
· Hydroelectric |
Examples:
· Chemical from Fossil Fuels
· Nuclear from Radioactive Materials
· Geothermal Energy |
| Renewable: theoretically an unlimited supply of these sources. |
Non-Renewable: Limited supply that will run out at some point. |
| High infrastructure setup costs as energy production methods are relatively new and under-researched. |
Cheap to setup and generate electricity as infrastructure of generation and supply has been in place for centuries. |
| Sources can be unreliable in their availability. E.g. no sunlight on cloudy days |
Energy production is exceptionally reliable and constantly availability. |
| Difficult to scale to large sizes. E.g. solar plants can span large areas for a low amount of energy generation. |
Easy to scale to larger applications. A single coal plant can produce the same energy as a much larger solar farm. |
| These sources do not release polluting greenhouse gasses into the atmosphere. |
Fossil Fuels are notorious for heavy air pollution with greenhouse gasses. |
| Quiet |
Geothermal power plants are loud, and Fracking is particularly harmful to the environment. |
| Low maintenance |
Nuclear Reactors are extremely high maintenance. |
| Low risk involved |
Global warming poses a huge risk to the future of humanity, while the risk of a Nuclear Fallout is ever-present with Nuclear power plants. |
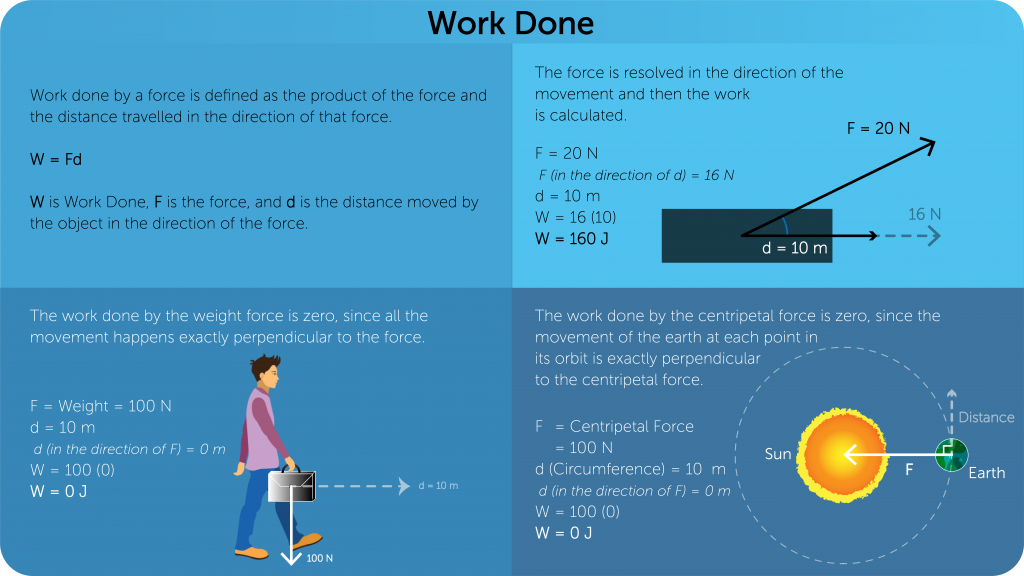
Work
- Work done in a process is defined as the amount of energy transferred by that process.
- Mechanical work is defined as the product of the magnitude of force on an object and the distance moved in the direction of the force by the object.
- Work done is a scalar quantity.
- SI Unit is Joules (J).
Power
- Power in its simplest definition is the work done or energy per unit time.
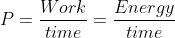
- Power is a scalar quantity.
- SI unit is Watt (W)
- The amount of Work done lifting a object into the air is
- The amount of power required changes drastically if this work was done over or .

