Structure of an Atom
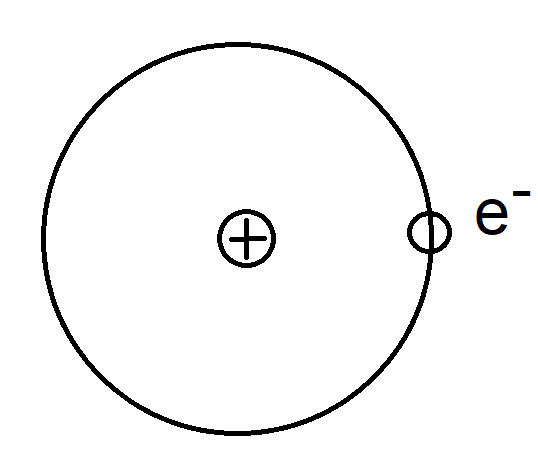
- An atom contains a positively charged nucleus surrounded by orbiting negatively charged electrons.
- An atom is electrically neutral
- Number of protons = Number of electrons
- Nucleus is Positively charged
- The nucleus contains nucleons, which are positively charged protons and neutral neutrons.
- Number of Nucleons (A) = Number of Protons (Z) + Number of Neutrons (N)
- Nuclide Notation for Element (X):
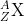
- An atom of an element that has a varying number of neutrons (same number of protons) in its nucleus is called an isotope of an element.
- A changes
- N changes
- Z remains the same
Rutherford’s Scattering Experiment
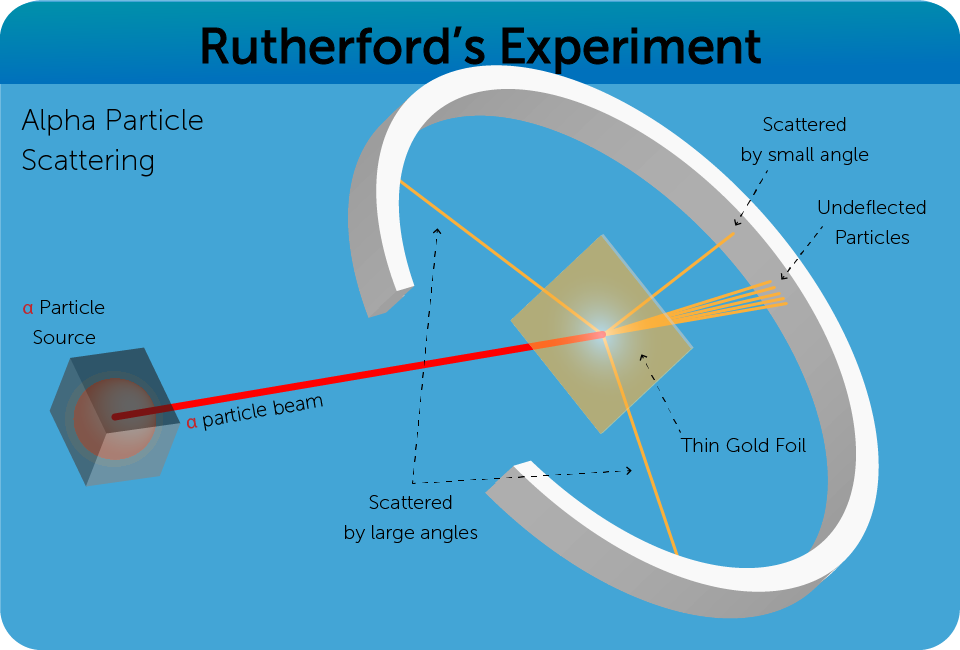
- The size of an Atom is much larger than the nucleus (approximately 100,000 times).
- This is proven by Rutherford’s experiment where a majority of
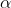 -particles (
-particles ( , Helium nucleus) go undeflected or deflect very little ( <
, Helium nucleus) go undeflected or deflect very little ( < 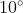 ) upon passing through a thin gold foil.
) upon passing through a thin gold foil.
- A very small number are deflected by large angles, more than
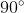 , which shows that only some of the positive
, which shows that only some of the positive 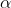 -particles interact with the positively charged Gold nucleus.
-particles interact with the positively charged Gold nucleus.
- The Gold nuclei impacted show a negligible recoil, implying that they are massive, containing a majority of the mass of the atom.
- These results imply that a majority of the atom is filled with empty space with a small nucleus and electrons orbiting it.
Nuclear Fission and Fusion
Nuclear Fission
- It is the splitting of a heavy nucleus into two intermediate-mass nuclei.
- A fission equation of the splitting of a Uranium nucleus (U-235) is as follows:

- A slow-moving neutron collides with the U-235 nucleus and triggers the fission reaction.
- Note that there are 3 neutrons released by the reaction, which go on to cause a chain reaction by colliding with other U-235 nuclei.
- Note that in the above nuclear equation, all the superscripts and subscripts add up to be the same on both sides.
Nuclear Fusion
- It is a nuclear reaction in which low-mass nuclei combine at very-high temperature and pressure to form an intermediate-mass nucleus.
- High temperatures are required to energize the nuclei enough to make them overcome electrical repulsion and fuse.

- Note that in the above nuclear equation, all the superscripts and subscripts add up to be the same on both sides.

